Global demand for energy is increasing by the hour as developing countries move toward industrialization. Experts estimate that by the year 2050, worldwide demand for electricity may reach 30 terawatts (TW). For perspective, one terawatt is roughly equal to the power of 1.3 billion horses.
Energy from the sun is limitless – the sun provides us 120,000 TW of power at any given instant – and it is free. But today solar energy provides only about one percent of the world’s electricity. The critical challenge is making it less expensive to convert photo-energy into usable electrical energy.
To do that, we need to find materials that absorb sunlight and convert it into electricity efficiently. In addition, we want these materials to be abundant, environmentally benign and cost-effective to fabricate into solar devices.
Researchers from around the world are working to develop solar cell technologies that are efficient and affordable. The goal is to bring the installation cost of solar electricity below US$1 per watt, compared to about $3 per watt today.
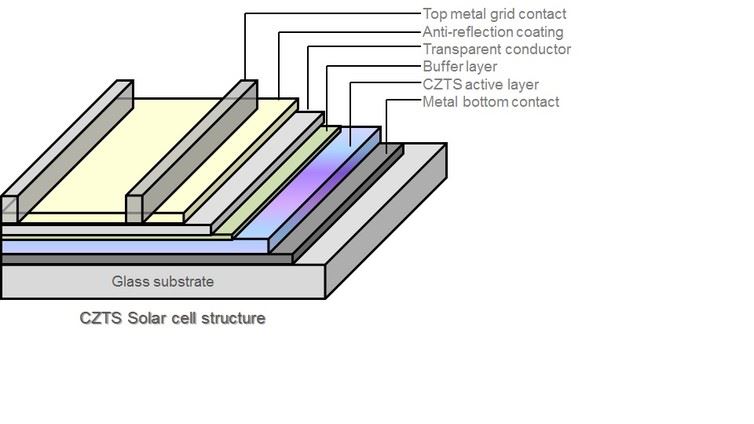
At Binghamton University’s Center for Autonomous Solar Power (CASP), we are investigating ways to make thin film solar cells using materials that are abundant in nature and nontoxic. We want to develop solar cells that are reliable, highly efficient at converting sunlight to electricity and inexpensive to manufacture. We have identified two materials that have great potential as solar absorbers: pyrite, better known as fool’s gold because of its metallic luster; and copper-zinc-tin-sulfide (CZTS).
Seeking the ideal material
Today’s commercial solar cells are made from one of three materials: silicon, cadmium telluride (CdTe) and copper-indium-gallium-selenide (CIGS). Each has strengths and weaknesses.
Silicon solar cells are highly efficient, converting up to 25 percent of the sunlight that falls on them into electricity, and very durable. However, it is very expensive to process silicon into wafers. And these wafers have to be very thick (about 0.3 millimeters, which is thick for solar cells) to absorb all of the sunlight that falls on them, which further increases costs.
Silicon solar cells – often referred to as first-generation solar cells – are used in the panels that have become familiar sights on rooftops. Our center is studying another type called thin film solar cells, which are the next generation of solar technology. As their name suggests, thin film solar cells are made by putting a thin layer of solar absorbent material over a substrate, such as glass or plastic, which typically can be flexible.

These solar cells use less material, so they are less expensive than crystalline solar cells made from silicon. It is not possible to coat crystalline silicon on a flexible substrate, so we need a different material to use as a solar absorber.
Although thin film solar technology is improving rapidly, some of the materials in today’s thin film solar cells are scarce or hazardous. For example, the cadmium in CdTe is highly toxic to all living things and is known to cause cancer in humans. CdTe can separate into cadmium and tellurium at high temperatures (for example, in a laboratory or housefire), posing a serious inhalation risk.
We are working with pyrite and CZTS because they are nontoxic and very inexpensive. CZTS costs about 0.005 cents per watt, and pyrite costs a mere 0.000002 cents per watt. They also are among the most abundant materials in the Earth’s crust, and absorb the visible spectrum of sunlight efficiently. These films can be as thin as 1/1000th of a millimeter.
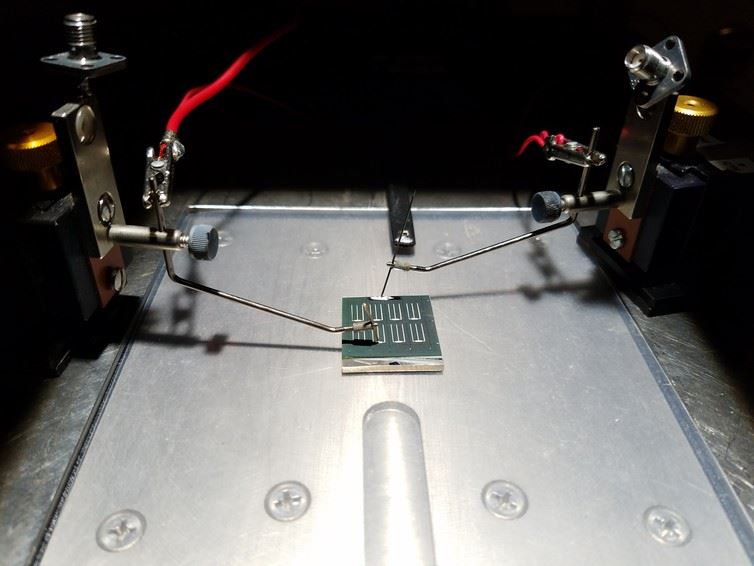
We need to crystallize these materials before we can fabricate them into solar cells. This is done by heating them. CZTS crystallizes at temperatures under 600 degree Celsius, compared to 1,200 degrees Celsius or higher for silicon, which makes it less expensive to process. It performs much like high-efficiency copper indium gallium selenide (CIGS) solar cells, which are commercially available now, but replaces the indium and gallium in these cells with cheaper and more abundant zinc and tin.
So far, however, CZTS solar cells are relatively inefficient: they convert less than 13 percentof the sunlight that falls upon them to electricity, compared to 20 percent for more expensive CIGS solar cells.
We know that CZTS solar cells have a potential to be 30 percent efficient. The main challenges are 1) synthesizing high-quality CZTS thin film without any traces of impurities, and 2) finding a suitable material for the “buffer” layer underneath it, which helps to collect the electric charges that sunlight creates in the absorber layer. Our lab has produced a CZTS thin film with seven percent efficiency; we hope to approach 15 percent efficiency soon by synthesizing high-quality CZTS layers and finding suitable buffer layers.
Pyrite is another potential absorber that can be synthesized at very low temperatures. Our lab has synthesized pyrite thin films, and now we are working to layer those films into solar cells. This process is challenging because pyrite breaks down easily when it is exposed to heat and moisture. We are researching ways to make it more stable without affecting its solar absorbency and mechanical properties. If we can solve this problem, “fool’s gold” could turn into a smart photovoltaic device.
In a recent study, researchers at Stanford University and the University of California at Berkeley estimated that solar power could provide up to 45 percent of U.S. electricity by 2050. To meet that target, we need to keep driving down the cost of solar power and find ways to make solar cells more sustainably. We believe that abundant, nontoxic materials are key to realizing the potential of solar power.









